|
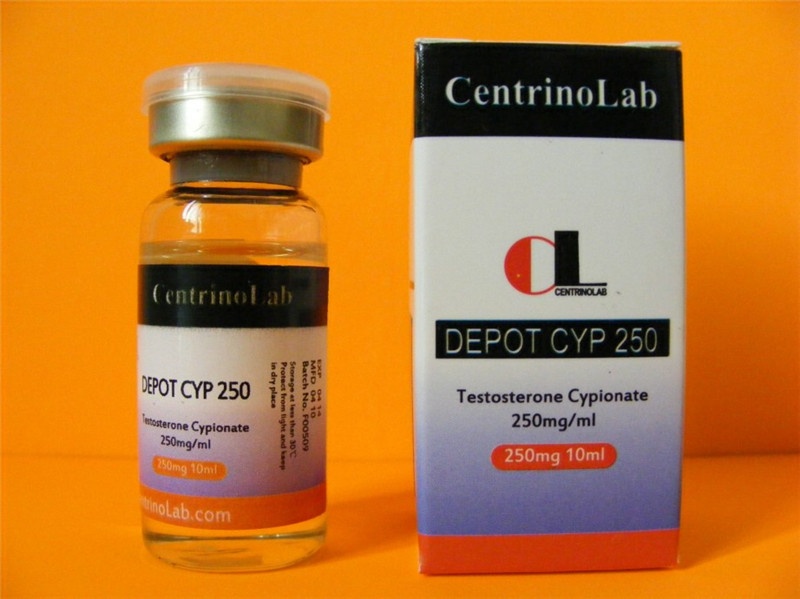
Package : 10ml vial contains 250mg/ml
Substance : Testosterone Cypionate
Profile : NomadLab ®Testosterone cypionate
INDICATIONS AND USAGE
Testosterone cypionate Injection is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
CONTRAINDICATIONS
1. Known hypersensitivity to the drug
2. Males with carcinoma of the breast
3. Males with known or suspected carcinoma of the prostate gland
4. Women who are or who may become pregnant
5. Patients with serious cardiac, hepatic or renal disease
WARNINGS
Hypercalcemia may occur in immobilized patients. If this occurs, the drug should be discontinued.
Prolonged use of high doses of androgens (principally the 17-α alkyl-androgens) has been associated with development of hepatic adenomas, hepatocellular carcinoma, and peliosis hepatis —all potentially life-threatening complications.
Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
Edema, with or without congestive heart failure, may be a serious complication in patients with pre-existing cardiac, renal or hepatic disease. Gynecomastia may develop and occasionally persists in patients being treated for hypogonadism.
This product contains benzyl alcohol. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants.
Androgen therapy should be used cautiously in healthy males with delayed puberty.
The effect on bone maturation should be monitored by assessing bone age of the wrist and hand every 6 months. In children, androgen treatment may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect may result in compromised adult stature.
This drug has not been shown to be safe and effective for the enhancement of athletic performance. Because of the potential risk of serious adverse health effects, this drug should not be used for such purpose.
PRECAUTIONS
General: Patients with benign prostatic hypertrophy may develop acute urethral obstruction.
Priapism or excessive sexual stimulation may develop. Oligospermia may occur after prolonged administration or excessive dosage. If any of these effects appear, the androgen should be stopped and if restarted, a lower dosage should be utilized.
Testosterone cypionate should not be used interchangeably with testosterone propionate because of differences in duration of action.
Testosterone cypionate is not for intravenous use.
Information for patients:
Patients should be instructed to report any of the following:
Nausea, vomiting, changes in skin color, ankle swelling, too frequent or persistent erections of the penis.
Laboratory tests:
Hemoglobin and hematocrit levels (to detect polycythemia) should be checked periodically in patients receiving long-term androgen administration.
Serum cholesterol may increase during androgen therapy.
Drug interactions:
Androgens may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may require reduction in order to maintain satisfactory therapeutic hypoprothrombinemia.
Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirements.
Drug/Laboratory test Interferences:
Androgens may decrease levels of thyroxinebinding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis:
Animal data.
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically- induced carcinomas of the liver in rats.
Human data.
There are rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases. Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
Pregnancy:
Teratogenic Effects. Pregnancy Category X. (See CONTRAINDICATIONS.)
Nursing mothers:
DEPO-Testosterone is not recommended for use in nursing mothers.
ADVERSE REACTIONS
The following adverse reactions in the male have occurred with some androgens:
Endocrine and urogenital: Gynecomastia and excessive frequency and duration of penile erections. Oligospermia may occur at high dosages.
Skin and appendages: Hirsutism, male pattern of baldness, seborrhea, and acne.
Fluid and electrolyte disturbances: Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates.
Gastrointestinal: Nausea, cholestatic jaundice, alterations in liver function tests, rarely hepatocellular neoplasms and peliosis hepatis (see WARNINGS).
Hematologic: Suppression of clotting factors II, V, VII, and X, bleeding in patients on concomitant anticoagulant therapy, and polycythemia.
Nervous system: Increased or decreased libido, headache, anxiety, depression, and generalized
paresthesia.
Allergic: Hypersensitivity, including skin manifestations and anaphylactoid reactions.
Miscellaneous: Inflammation and pain at the site of intramuscular injection.
OVERDOSAGE
There have been no reports of acute overdosage with the androgens.
DOSAGE AND ADMINISTRATION
DEPO-Testosterone Injection is for intramuscular use only.
It should not be given intravenously. Intramuscular injections should be given deep in the gluteal muscle.
The suggested dosage for DEPO-Testosterone Injection varies depending on the age, sex, and diagnosis of the individual patient. Dosage is adjusted according to the patient’s response and the appearance of adverse reactions.
Various dosage regimens have been used to induce pubertal changes in hypogonadal males; some experts have advocated lower dosages initially, gradually increasing the dose as puberty progresses, with or without a decrease to maintenance levels. Other experts emphasize that higher dosages are needed to induce pubertal changes and lower dosages can be used for maintenance after puberty. The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose.
For replacement in the hypogonadal male, 50-400 mg should be administered every two to four weeks.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Warming and shaking the vial should redissolve any crystals that may have formed during storage at temperatures lower than recommended.
Vials should be stored at controlled room temperature 20° to 25°C (68° to 77°F)
Protect from light.
|




 Bestropin
Bestropin