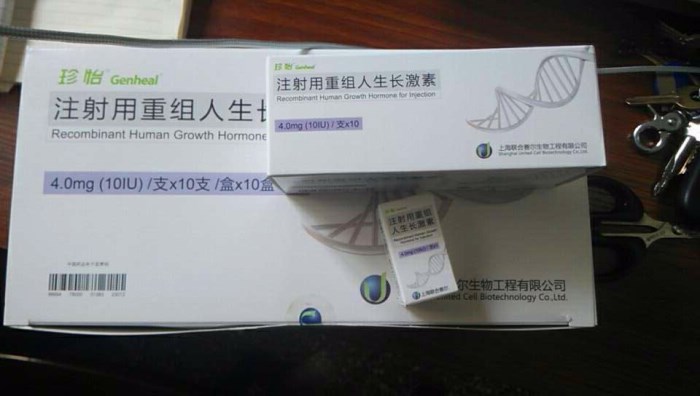
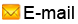Genheal™ (somatropin [rDNA origin] for injection) is one of the leading rHGH in China that has been widely used in more than 300 Class A hospitals for pediatric, burn, surgery, epidemiology and other areas of clinical treatment. Genheal™ conform both domestic and international quality standards and is one of the best rHGH products on the market today.
Genheal™ is approved by the Food and Drug Administration (FDA) for the following indications:
• Growth hormone deficiency in children with long-term treatment
• Treatment of metabolic diseases and AIDS-related wasting
• Prader –Willi syndrome caused by long-term treatment of children with growth deficiencies
• Continued after birth, intrauterine growth retardation short stature
• Adults with growth hormone deficiency (GHD)
• Long-term treatment of patients with Turner syndrome
• Idiopathic short stature
• Short bowel syndrome
• Pediatric renal transplantation before renal failure related to lack of growth
• SHOX gene deficiency
Genheal™ is manufactured using Protein Secretion Technology- the most advanced rHGH manufacturing technology. Its major advantage is that rHGH is secreted from E. coli and does not accumulate in inclusion bodies. This process allows for easier purification of rHGH and the production of rHGH that is identical to human growth hormone.
High quality standards has determined strong overseas demand for rHGH so the decision to lunch the export version of Genheal™ was made. Genheal™ has been exported to Southeast Asia, India, South America, Middle East, Eastern Europe and other countries and regions.
Genheal™ for export is dosed in the following forms:
4mg (10IU) vial/x 10iu



 Bestropin
Bestropin